In the next generation of advanced secondary battery technology, all-solid-state lithium batteries and lithium-sulfur batteries are the two main development systems. Sulfide all-solid-state batteries use safe, non-flammable solid electrolytes. This helps fix safety issues found in batteries with organic electrolytes. Lithium-sulfur batteries feature lithium metal as the negative electrode. They use sulfur or lithium sulfide (Li2S) for the positive electrode. These batteries can store energy that is five times greater than regular lithium-ion batteries. In recent years, these two battery types have become key in energy tech development worldwide.

What is lithium sulfide (Li2S)?
Lithium sulfide (Li2S) is essential for making sulfide solid electrolytes (SSE). It is also the top choice for the positive electrode in lithium-sulfur batteries. Using negative electrodes like graphite and silicon can help prevent safety risks tied to lithium metal electrodes. So, the demand for Li2S materials has been rising in the global market lately.
Before lithium-sulfur and sulfide all-solid-state batteries gained attention, lithium sulfide had few practical uses. Not many people studied it at that time. The editor of China Powder Network checked the information. They found that in the 20th century, only a few Chinese documents discussed Li2S in batteries. The first was a research report from Academician Chen Liquan at the Institute of Physics, Chinese Academy of Sciences, along with researchers from Peking University in 1985. After almost 30 years, around 2015, the research enthusiasm for lithium sulfide gradually heated up. Today, lithium sulfide is a “star” in all-solid-state batteries and lithium-sulfur materials.
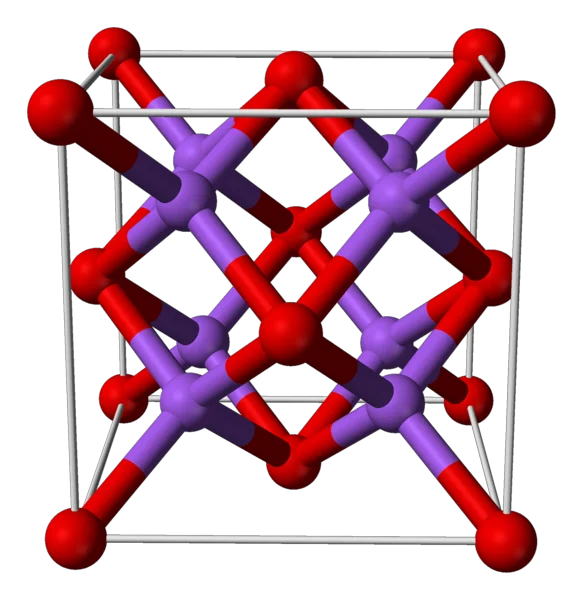
How to prepare lithium sulfide (Li2S)?
Pure lithium sulfide appears as white to yellow crystals. It has an antifluorite structure. Its relative density is 1.66. The melting point is 938°C, and the boiling point is 1372°C. It dissolves easily in water and is also soluble in ethanol and acid. However, it does not dissolve in alkali. Li2S compounds don’t exist in nature. They break down easily in air, which leads to the production of hydrogen sulfide gas. This gas has a smell like rotten eggs.
Unlike the first, the other two alkali metal sulfides, Na2S and K2S, can mix with water. They form hydrated crystals: Na2S·9H2O and K2S·5H2O. You can get their anhydrous forms by heating them directly. These three sulfides have very similar chemical properties. They share the same divalent anion, S2-. This similarity makes them useful in papermaking, leather making, and rubber vulcanization. Li2S costs much more than the other two similar products. This is mainly because lithium, its raw material, is expensive. Also, production and storage are challenging.
Current methods for making lithium sulfide include:
- Ball milling method
- Solvent method
- High temperature and high pressure method
- Direct carbon composite method
Ball milling
Process principle: Mix elemental sulfur with metallic lithium or lithium hydride in an inert atmosphere. Then, use mechanical ball milling to create lithium sulfide.
Advantages: Simple process, environmentally friendly, no waste liquid generated.
Disadvantages:
- High cost of raw materials (lithium hydride)
- Long reaction time
- Low conversion rate
- The product contains impurities like lithium polysulfide, which are hard to purify
- Choosing industrial equipment is challenging.
Solvent method
Process principle: Mix lithium and sulfur compounds in a solvent. This reaction makes lithium sulfide. The solvent can be an organic solvent or liquid ammonia. Organic solvents include aliphatic hydrocarbons, aromatic hydrocarbons, or ether solvents. Common examples are ethanol, hexane, toluene, ether, tetrahydrofuran, and nitrogen methyl pyrrolidone.
Advantages:
- The liquid phase reaction is complete.
- Impurities are less likely to stay.
- Purifying the product is easy.
- No high temperature treatment is needed.
- Energy consumption is low.
- The process is simple, and working conditions are easy to control.
Disadvantages: Organic solvents can catch fire, explode, and evaporate quickly. This leads to serious pollution and makes recycling hard. Also, working with them is very dangerous and tough to manage.
High temperature and high pressure method
Process principle: In a protective atmosphere that is inert or reducing, high temperature and pressure make lithium and sulfur compounds react. This reaction helps create lithium sulfide.
Advantages: The process is simple. It generates no harmful gas. It also uses high temperature and pressure to prevent harmful solvent leaks. This greatly shortens the preparation time.
Disadvantages:
- High temperature and pressure make control tough.
- There are strict equipment requirements.
- The reaction process and post-processing carry increased risks.
Direct Carbon Composite Process
Process principle: Carbon can easily reduce materials. This lets us add carbon directly when making lithium sulfide. This makes a lithium sulfide/carbon composite. It has even dispersion, strong performance, and a controllable shape, all in one step.
Advantages:
- The reaction is easier to control. This helps with the production and storage issues caused by lithium sulfide’s sensitivity to water and oxygen.
- It boosts product yield and performance.
- It simplifies the complex preparation process of traditional lithium sulfide/carbon composites.
- It improves how active materials spread in the positive electrode of lithium-sulfur batteries.
- It also improves the electrochemical performance of these batteries.
Disadvantages: The process technology needs improvement. The product quality is unstable. Also, the morphology of the composite material is hard to control.
What is the difficulty in industrializing battery-grade lithium sulfide (Li2S)?
Lithium sulfide is a key raw material for sulfide solid electrolytes. Japan, South Korea, the United States, and China lead in battery technology. Japan and South Korea are pushing hard for sulfur-based solid-state batteries. They are making strategic plans to advance this technology. Although all companies are vigorously promoting the development of sulfur-based solid-state batteries, sulfur-based solid-state batteries have not yet been industrialized.
The main reasons are two points:
- The high cost of raw materials, especially lithium sulfide.
- The interface problem in sulfur-based all-solid-state batteries impacts the link between the positive and negative electrodes and the solid electrolyte.
Lithium sulfide is important for making sulfide solid electrolytes and for lithium-sulfur batteries. Its purity, particle size, and shape are crucial for the battery’s performance. Production cost is also key for successfully using lithium sulfide in commercial batteries.
High-risk raw materials are difficult to obtain
The main materials for lithium sulfide are:
- Metallic lithium or lithium hydride
- Hydrogen sulfide gas
- Organic solvents
Metallic lithium and lithium hydride can be hard to find, and H2S is a very toxic gas. This makes it dangerous to transport, use, and store. Most organic solvents are flammable or explosive hazardous chemicals. There are many uncertainties and risks in getting and storing lithium sulfide raw materials.
The separation and purification of high-purity products is difficult
Some electrolyte manufacturers now demand higher purity for lithium sulfide. They set these standards:
- Carbon content must be 0.1% or lower.
- Moisture content should be ≤100 mg/kg.
- Metal impurities must be under 100 mg/kg.
- Particle size must be D50≤7 um and D90≤2D50.
Some lithium sulfide makers must purify their product. This adds complexity to the process.
The development of special equipment for lithium sulfide production is difficult
Lithium sulfide is a new lithium salt. It is an important raw material for the next generation of high-energy-density solid-state batteries. Right now, China is working on turning this material from small-scale production to full industrial use. Industrial equipment needs to be customized according to the process, and the equipment development is difficult. The current material system fails to meet the customization needs for lithium sulfide industrial equipment. The main issue holding back lithium sulfide industrialization is the need for special equipment.